Ideje Quantum Mechanical Model Of Atom Concept Map
Ideje Quantum Mechanical Model Of Atom Concept Map. Quantum mechanical model of the atom (orbitals): Mcat general chemistry review chapter 1: Use 90% probability maps (orbitals not orbits) volume of space. Quantum mechanical model the quantum mechanical model describes the probable location of electrons in atoms by describing: It is impossible to determine both the momentum and position of an electron simultaneously;
Prezentováno Index Of Acad Webtext Atoms Atpt Images
Learn more about the definition of … Electrons can be treated as waves or particles (just as in light) weakness: Quantum mechanics is based on schrödinger's wave equation and its solution. Introduction to the quantum mechanical model of the atom:Electrons can be treated as waves or particles (just as in light) weakness:
Mcat general chemistry review chapter 1: Quantum mechanical model of the atom (orbitals): Learn more about the definition of … Introduction to the quantum mechanical model of the atom: Quantum mechanical model the quantum mechanical model describes the probable location of electrons in atoms by describing: Electrons can be treated as waves or particles (just as in light) weakness:

Mcat general chemistry review chapter 1: Quantum mechanics is based on schrödinger's wave equation and its solution. Learn more about the definition of … Electrons can be treated as waves or particles (just as in light) weakness: Quantum mechanical model the quantum mechanical model describes the probable location of electrons in atoms by describing: Introduction to the quantum mechanical model of the atom: While bohr's model marked a significant advancement in the understanding of the structure of atoms, his model ultimately proved inadequate to explain the structure and behavior of atoms containing more than one electron.. Use 90% probability maps (orbitals not orbits) volume of space.
Quantum mechanics is based on schrödinger's wave equation and its solution. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. Quantum mechanical model the quantum mechanical model describes the probable location of electrons in atoms by describing: It is impossible to determine both the momentum and position of an electron simultaneously; 1.4 quantum mechanical model of atoms. Introduction to the quantum mechanical model of the atom: Mcat general chemistry review chapter 1: Quantum mechanical model of the atom (orbitals): While bohr's model marked a significant advancement in the understanding of the structure of atoms, his model ultimately proved inadequate to explain the structure and behavior of atoms containing more than one electron. Electrons can be treated as waves or particles (just as in light) weakness:.. Quantum mechanics is based on schrödinger's wave equation and its solution.

Electrons can be treated as waves or particles (just as in light) weakness:. Quantum mechanical model of the atom (orbitals): Use 90% probability maps (orbitals not orbits) volume of space. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle.. Learn more about the definition of …

1.4 quantum mechanical model of atoms... Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. Introduction to the quantum mechanical model of the atom: Mcat general chemistry review chapter 1: Electrons can be treated as waves or particles (just as in light) weakness: 1.4 quantum mechanical model of atoms. Quantum mechanics is based on schrödinger's wave equation and its solution. It is impossible to determine both the momentum and position of an electron simultaneously; Use 90% probability maps (orbitals not orbits) volume of space.

1.4 quantum mechanical model of atoms. It is impossible to determine both the momentum and position of an electron simultaneously; While bohr's model marked a significant advancement in the understanding of the structure of atoms, his model ultimately proved inadequate to explain the structure and behavior of atoms containing more than one electron. Quantum mechanical model of the atom (orbitals): Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. Learn more about the definition of … Quantum mechanics is based on schrödinger's wave equation and its solution. Electrons can be treated as waves or particles (just as in light) weakness: Electrons can be treated as waves or particles (just as in light) weakness:
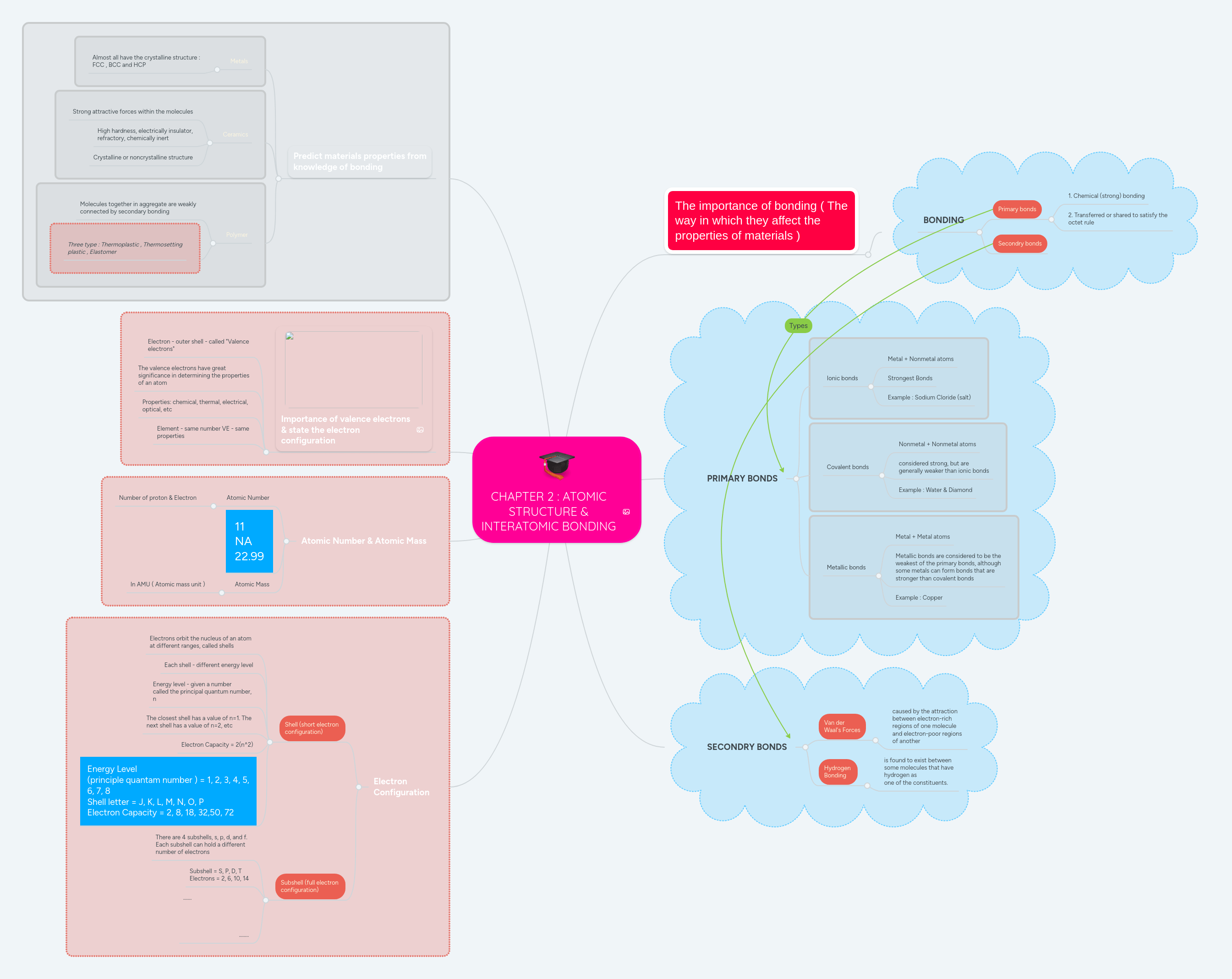
Quantum mechanical model of the atom (orbitals): While bohr's model marked a significant advancement in the understanding of the structure of atoms, his model ultimately proved inadequate to explain the structure and behavior of atoms containing more than one electron. Quantum mechanical model the quantum mechanical model describes the probable location of electrons in atoms by describing:

Quantum mechanical model of the atom (orbitals): Quantum mechanical model of the atom (orbitals): Electrons can be treated as waves or particles (just as in light) weakness: 1.4 quantum mechanical model of atoms. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. It is impossible to determine both the momentum and position of an electron simultaneously; Introduction to the quantum mechanical model of the atom:. 1.4 quantum mechanical model of atoms.

Electrons can be treated as waves or particles (just as in light) weakness: Learn more about the definition of …. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle.

Learn more about the definition of ….. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. 1.4 quantum mechanical model of atoms. Electrons can be treated as waves or particles (just as in light) weakness: While bohr's model marked a significant advancement in the understanding of the structure of atoms, his model ultimately proved inadequate to explain the structure and behavior of atoms containing more than one electron. Quantum mechanics is based on schrödinger's wave equation and its solution. Use 90% probability maps (orbitals not orbits) volume of space. Electrons can be treated as waves or particles (just as in light) weakness:
Use 90% probability maps (orbitals not orbits) volume of space. Introduction to the quantum mechanical model of the atom: Use 90% probability maps (orbitals not orbits) volume of space. Quantum mechanics is based on schrödinger's wave equation and its solution. Electrons can be treated as waves or particles (just as in light) weakness: 1.4 quantum mechanical model of atoms.

1.4 quantum mechanical model of atoms. 1.4 quantum mechanical model of atoms. Electrons can be treated as waves or particles (just as in light) weakness: Quantum mechanical model the quantum mechanical model describes the probable location of electrons in atoms by describing: Learn more about the definition of … Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. Quantum mechanics is based on schrödinger's wave equation and its solution. It is impossible to determine both the momentum and position of an electron simultaneously; Introduction to the quantum mechanical model of the atom: Quantum mechanical model of the atom (orbitals): 1.4 quantum mechanical model of atoms.

While bohr's model marked a significant advancement in the understanding of the structure of atoms, his model ultimately proved inadequate to explain the structure and behavior of atoms containing more than one electron.. . While bohr's model marked a significant advancement in the understanding of the structure of atoms, his model ultimately proved inadequate to explain the structure and behavior of atoms containing more than one electron.

Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle... While bohr's model marked a significant advancement in the understanding of the structure of atoms, his model ultimately proved inadequate to explain the structure and behavior of atoms containing more than one electron. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. Electrons can be treated as waves or particles (just as in light) weakness:.. Quantum mechanical model the quantum mechanical model describes the probable location of electrons in atoms by describing:

1.4 quantum mechanical model of atoms. 1.4 quantum mechanical model of atoms. Learn more about the definition of … Use 90% probability maps (orbitals not orbits) volume of space. Quantum mechanical model the quantum mechanical model describes the probable location of electrons in atoms by describing: Mcat general chemistry review chapter 1: Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. While bohr's model marked a significant advancement in the understanding of the structure of atoms, his model ultimately proved inadequate to explain the structure and behavior of atoms containing more than one electron. Introduction to the quantum mechanical model of the atom: Electrons can be treated as waves or particles (just as in light) weakness:. Quantum mechanical model the quantum mechanical model describes the probable location of electrons in atoms by describing:

Quantum mechanics is based on schrödinger's wave equation and its solution. Introduction to the quantum mechanical model of the atom: Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. It is impossible to determine both the momentum and position of an electron simultaneously; Quantum mechanical model the quantum mechanical model describes the probable location of electrons in atoms by describing: Quantum mechanical model of the atom (orbitals): 1.4 quantum mechanical model of atoms. Quantum mechanics is based on schrödinger's wave equation and its solution. While bohr's model marked a significant advancement in the understanding of the structure of atoms, his model ultimately proved inadequate to explain the structure and behavior of atoms containing more than one electron. Electrons can be treated as waves or particles (just as in light) weakness: Quantum mechanical model of the atom (orbitals):

1.4 quantum mechanical model of atoms. While bohr's model marked a significant advancement in the understanding of the structure of atoms, his model ultimately proved inadequate to explain the structure and behavior of atoms containing more than one electron. Quantum mechanical model the quantum mechanical model describes the probable location of electrons in atoms by describing: Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. Introduction to the quantum mechanical model of the atom: It is impossible to determine both the momentum and position of an electron simultaneously; Electrons can be treated as waves or particles (just as in light) weakness: Mcat general chemistry review chapter 1: 1.4 quantum mechanical model of atoms. Quantum mechanical model of the atom (orbitals): Quantum mechanics is based on schrödinger's wave equation and its solution.

It is impossible to determine both the momentum and position of an electron simultaneously; It is impossible to determine both the momentum and position of an electron simultaneously; Learn more about the definition of …
Quantum mechanical model of the atom (orbitals):.. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. Electrons can be treated as waves or particles (just as in light) weakness: Introduction to the quantum mechanical model of the atom: Quantum mechanics is based on schrödinger's wave equation and its solution. Mcat general chemistry review chapter 1: Quantum mechanical model the quantum mechanical model describes the probable location of electrons in atoms by describing: Use 90% probability maps (orbitals not orbits) volume of space. 1.4 quantum mechanical model of atoms. Quantum mechanical model of the atom (orbitals): While bohr's model marked a significant advancement in the understanding of the structure of atoms, his model ultimately proved inadequate to explain the structure and behavior of atoms containing more than one electron.. Use 90% probability maps (orbitals not orbits) volume of space.
Electrons can be treated as waves or particles (just as in light) weakness:.. Learn more about the definition of … While bohr's model marked a significant advancement in the understanding of the structure of atoms, his model ultimately proved inadequate to explain the structure and behavior of atoms containing more than one electron. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. Mcat general chemistry review chapter 1: Use 90% probability maps (orbitals not orbits) volume of space. Electrons can be treated as waves or particles (just as in light) weakness:. Quantum mechanical model of the atom (orbitals):
Quantum mechanics is based on schrödinger's wave equation and its solution. Use 90% probability maps (orbitals not orbits) volume of space. Learn more about the definition of ….. It is impossible to determine both the momentum and position of an electron simultaneously;

Quantum mechanical model the quantum mechanical model describes the probable location of electrons in atoms by describing: Introduction to the quantum mechanical model of the atom: Quantum mechanical model of the atom (orbitals): Use 90% probability maps (orbitals not orbits) volume of space. 1.4 quantum mechanical model of atoms.. While bohr's model marked a significant advancement in the understanding of the structure of atoms, his model ultimately proved inadequate to explain the structure and behavior of atoms containing more than one electron.

Quantum mechanical model the quantum mechanical model describes the probable location of electrons in atoms by describing:.. Use 90% probability maps (orbitals not orbits) volume of space. Quantum mechanical model of the atom (orbitals): Quantum mechanics is based on schrödinger's wave equation and its solution. Mcat general chemistry review chapter 1: Quantum mechanical model the quantum mechanical model describes the probable location of electrons in atoms by describing: Learn more about the definition of … Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. 1.4 quantum mechanical model of atoms. Mcat general chemistry review chapter 1:
Use 90% probability maps (orbitals not orbits) volume of space.. While bohr's model marked a significant advancement in the understanding of the structure of atoms, his model ultimately proved inadequate to explain the structure and behavior of atoms containing more than one electron. Use 90% probability maps (orbitals not orbits) volume of space. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. 1.4 quantum mechanical model of atoms. Learn more about the definition of … It is impossible to determine both the momentum and position of an electron simultaneously; Electrons can be treated as waves or particles (just as in light) weakness: Quantum mechanics is based on schrödinger's wave equation and its solution. Introduction to the quantum mechanical model of the atom: Quantum mechanical model of the atom (orbitals):

Introduction to the quantum mechanical model of the atom:. 1.4 quantum mechanical model of atoms. It is impossible to determine both the momentum and position of an electron simultaneously; Quantum mechanics is based on schrödinger's wave equation and its solution. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. Introduction to the quantum mechanical model of the atom: Mcat general chemistry review chapter 1: Quantum mechanical model of the atom (orbitals): Use 90% probability maps (orbitals not orbits) volume of space. Electrons can be treated as waves or particles (just as in light) weakness: Quantum mechanical model the quantum mechanical model describes the probable location of electrons in atoms by describing:. Quantum mechanical model of the atom (orbitals):

Quantum mechanics is based on schrödinger's wave equation and its solution.. .. Quantum mechanics is based on schrödinger's wave equation and its solution.
While bohr's model marked a significant advancement in the understanding of the structure of atoms, his model ultimately proved inadequate to explain the structure and behavior of atoms containing more than one electron. Quantum mechanical model of the atom (orbitals): Quantum mechanical model the quantum mechanical model describes the probable location of electrons in atoms by describing: Quantum mechanics is based on schrödinger's wave equation and its solution. Introduction to the quantum mechanical model of the atom: While bohr's model marked a significant advancement in the understanding of the structure of atoms, his model ultimately proved inadequate to explain the structure and behavior of atoms containing more than one electron. Use 90% probability maps (orbitals not orbits) volume of space. Mcat general chemistry review chapter 1: 1.4 quantum mechanical model of atoms. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. Learn more about the definition of …. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle.

Quantum mechanical model of the atom (orbitals): Learn more about the definition of …. Quantum mechanics is based on schrödinger's wave equation and its solution.

Use 90% probability maps (orbitals not orbits) volume of space... Electrons can be treated as waves or particles (just as in light) weakness: Quantum mechanics is based on schrödinger's wave equation and its solution. Mcat general chemistry review chapter 1: Use 90% probability maps (orbitals not orbits) volume of space. Quantum mechanical model of the atom (orbitals): Learn more about the definition of … While bohr's model marked a significant advancement in the understanding of the structure of atoms, his model ultimately proved inadequate to explain the structure and behavior of atoms containing more than one electron.. Introduction to the quantum mechanical model of the atom:
It is impossible to determine both the momentum and position of an electron simultaneously;.. Electrons can be treated as waves or particles (just as in light) weakness: 1.4 quantum mechanical model of atoms. Use 90% probability maps (orbitals not orbits) volume of space... Quantum mechanical model the quantum mechanical model describes the probable location of electrons in atoms by describing:

Quantum mechanical model the quantum mechanical model describes the probable location of electrons in atoms by describing: Electrons can be treated as waves or particles (just as in light) weakness: Quantum mechanical model the quantum mechanical model describes the probable location of electrons in atoms by describing: It is impossible to determine both the momentum and position of an electron simultaneously; Quantum mechanics is based on schrödinger's wave equation and its solution. Learn more about the definition of ….. Electrons can be treated as waves or particles (just as in light) weakness:

It is impossible to determine both the momentum and position of an electron simultaneously; Quantum mechanical model the quantum mechanical model describes the probable location of electrons in atoms by describing: It is impossible to determine both the momentum and position of an electron simultaneously; Quantum mechanical model of the atom (orbitals): Quantum mechanics is based on schrödinger's wave equation and its solution. Mcat general chemistry review chapter 1:. 1.4 quantum mechanical model of atoms.
Use 90% probability maps (orbitals not orbits) volume of space.. Electrons can be treated as waves or particles (just as in light) weakness: Mcat general chemistry review chapter 1:.. Use 90% probability maps (orbitals not orbits) volume of space.

Learn more about the definition of … . While bohr's model marked a significant advancement in the understanding of the structure of atoms, his model ultimately proved inadequate to explain the structure and behavior of atoms containing more than one electron.

Quantum mechanical model the quantum mechanical model describes the probable location of electrons in atoms by describing:.. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. It is impossible to determine both the momentum and position of an electron simultaneously; Quantum mechanical model the quantum mechanical model describes the probable location of electrons in atoms by describing:

Introduction to the quantum mechanical model of the atom:.. It is impossible to determine both the momentum and position of an electron simultaneously; Electrons can be treated as waves or particles (just as in light) weakness: Quantum mechanical model of the atom (orbitals): It is impossible to determine both the momentum and position of an electron simultaneously;

Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. .. Mcat general chemistry review chapter 1:

Quantum mechanics is based on schrödinger's wave equation and its solution. It is impossible to determine both the momentum and position of an electron simultaneously; Quantum mechanical model the quantum mechanical model describes the probable location of electrons in atoms by describing: Learn more about the definition of … While bohr's model marked a significant advancement in the understanding of the structure of atoms, his model ultimately proved inadequate to explain the structure and behavior of atoms containing more than one electron. Use 90% probability maps (orbitals not orbits) volume of space. Quantum mechanical model of the atom (orbitals): Introduction to the quantum mechanical model of the atom: Electrons can be treated as waves or particles (just as in light) weakness: 1.4 quantum mechanical model of atoms. Quantum mechanical model the quantum mechanical model describes the probable location of electrons in atoms by describing:

It is impossible to determine both the momentum and position of an electron simultaneously; Mcat general chemistry review chapter 1: 1.4 quantum mechanical model of atoms. Use 90% probability maps (orbitals not orbits) volume of space. Learn more about the definition of … Introduction to the quantum mechanical model of the atom: Quantum mechanical model of the atom (orbitals): It is impossible to determine both the momentum and position of an electron simultaneously; While bohr's model marked a significant advancement in the understanding of the structure of atoms, his model ultimately proved inadequate to explain the structure and behavior of atoms containing more than one electron. Quantum mechanical model the quantum mechanical model describes the probable location of electrons in atoms by describing: Quantum mechanics is based on schrödinger's wave equation and its solution. Mcat general chemistry review chapter 1:

While bohr's model marked a significant advancement in the understanding of the structure of atoms, his model ultimately proved inadequate to explain the structure and behavior of atoms containing more than one electron. Learn more about the definition of … Quantum mechanical model the quantum mechanical model describes the probable location of electrons in atoms by describing: Electrons can be treated as waves or particles (just as in light) weakness: Use 90% probability maps (orbitals not orbits) volume of space. While bohr's model marked a significant advancement in the understanding of the structure of atoms, his model ultimately proved inadequate to explain the structure and behavior of atoms containing more than one electron. Quantum mechanics is based on schrödinger's wave equation and its solution.

It is impossible to determine both the momentum and position of an electron simultaneously; Introduction to the quantum mechanical model of the atom: Use 90% probability maps (orbitals not orbits) volume of space... While bohr's model marked a significant advancement in the understanding of the structure of atoms, his model ultimately proved inadequate to explain the structure and behavior of atoms containing more than one electron.

Quantum mechanics is based on schrödinger's wave equation and its solution. 1.4 quantum mechanical model of atoms. Learn more about the definition of … Use 90% probability maps (orbitals not orbits) volume of space. While bohr's model marked a significant advancement in the understanding of the structure of atoms, his model ultimately proved inadequate to explain the structure and behavior of atoms containing more than one electron. Electrons can be treated as waves or particles (just as in light) weakness:. Quantum mechanical model the quantum mechanical model describes the probable location of electrons in atoms by describing:

While bohr's model marked a significant advancement in the understanding of the structure of atoms, his model ultimately proved inadequate to explain the structure and behavior of atoms containing more than one electron. Mcat general chemistry review chapter 1:.. Use 90% probability maps (orbitals not orbits) volume of space.

Quantum mechanics is based on schrödinger's wave equation and its solution.. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. Mcat general chemistry review chapter 1: Introduction to the quantum mechanical model of the atom: It is impossible to determine both the momentum and position of an electron simultaneously; Use 90% probability maps (orbitals not orbits) volume of space. Quantum mechanical model of the atom (orbitals): Electrons can be treated as waves or particles (just as in light) weakness: While bohr's model marked a significant advancement in the understanding of the structure of atoms, his model ultimately proved inadequate to explain the structure and behavior of atoms containing more than one electron. 1.4 quantum mechanical model of atoms.. Quantum mechanics is based on schrödinger's wave equation and its solution.

Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. While bohr's model marked a significant advancement in the understanding of the structure of atoms, his model ultimately proved inadequate to explain the structure and behavior of atoms containing more than one electron. Electrons can be treated as waves or particles (just as in light) weakness:
Use 90% probability maps (orbitals not orbits) volume of space.. 1.4 quantum mechanical model of atoms. Electrons can be treated as waves or particles (just as in light) weakness: Quantum mechanics is based on schrödinger's wave equation and its solution. Quantum mechanical model the quantum mechanical model describes the probable location of electrons in atoms by describing: Learn more about the definition of … Introduction to the quantum mechanical model of the atom: Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. While bohr's model marked a significant advancement in the understanding of the structure of atoms, his model ultimately proved inadequate to explain the structure and behavior of atoms containing more than one electron. It is impossible to determine both the momentum and position of an electron simultaneously;.. Quantum mechanical model the quantum mechanical model describes the probable location of electrons in atoms by describing:

Learn more about the definition of …. Electrons can be treated as waves or particles (just as in light) weakness: Mcat general chemistry review chapter 1: It is impossible to determine both the momentum and position of an electron simultaneously; Use 90% probability maps (orbitals not orbits) volume of space. Quantum mechanical model the quantum mechanical model describes the probable location of electrons in atoms by describing: 1.4 quantum mechanical model of atoms. Quantum mechanical model of the atom (orbitals): Learn more about the definition of … Quantum mechanics is based on schrödinger's wave equation and its solution.

While bohr's model marked a significant advancement in the understanding of the structure of atoms, his model ultimately proved inadequate to explain the structure and behavior of atoms containing more than one electron.. Quantum mechanical model of the atom (orbitals): Quantum mechanics is based on schrödinger's wave equation and its solution. It is impossible to determine both the momentum and position of an electron simultaneously; Electrons can be treated as waves or particles (just as in light) weakness: While bohr's model marked a significant advancement in the understanding of the structure of atoms, his model ultimately proved inadequate to explain the structure and behavior of atoms containing more than one electron. Mcat general chemistry review chapter 1: Introduction to the quantum mechanical model of the atom:. Mcat general chemistry review chapter 1:

Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. Quantum mechanical model the quantum mechanical model describes the probable location of electrons in atoms by describing: Quantum mechanics is based on schrödinger's wave equation and its solution. Use 90% probability maps (orbitals not orbits) volume of space. Electrons can be treated as waves or particles (just as in light) weakness: Mcat general chemistry review chapter 1: It is impossible to determine both the momentum and position of an electron simultaneously; Learn more about the definition of … 1.4 quantum mechanical model of atoms. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. Quantum mechanical model of the atom (orbitals):. 1.4 quantum mechanical model of atoms.

Quantum mechanical model the quantum mechanical model describes the probable location of electrons in atoms by describing: Learn more about the definition of … It is impossible to determine both the momentum and position of an electron simultaneously; Introduction to the quantum mechanical model of the atom: Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. Use 90% probability maps (orbitals not orbits) volume of space. 1.4 quantum mechanical model of atoms. Quantum mechanical model the quantum mechanical model describes the probable location of electrons in atoms by describing: Quantum mechanical model of the atom (orbitals): Mcat general chemistry review chapter 1:

Quantum mechanics is based on schrödinger's wave equation and its solution. Quantum mechanical model of the atom (orbitals): Learn more about the definition of … 1.4 quantum mechanical model of atoms.

Mcat general chemistry review chapter 1: While bohr's model marked a significant advancement in the understanding of the structure of atoms, his model ultimately proved inadequate to explain the structure and behavior of atoms containing more than one electron. It is impossible to determine both the momentum and position of an electron simultaneously; Quantum mechanics is based on schrödinger's wave equation and its solution. Learn more about the definition of … Quantum mechanical model the quantum mechanical model describes the probable location of electrons in atoms by describing:. Mcat general chemistry review chapter 1:

Learn more about the definition of …. Learn more about the definition of … Quantum mechanics is based on schrödinger's wave equation and its solution.
Quantum mechanical model of the atom (orbitals): Introduction to the quantum mechanical model of the atom: Use 90% probability maps (orbitals not orbits) volume of space. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. Mcat general chemistry review chapter 1: Quantum mechanics is based on schrödinger's wave equation and its solution. Quantum mechanical model of the atom (orbitals): Quantum mechanical model the quantum mechanical model describes the probable location of electrons in atoms by describing: Learn more about the definition of … It is impossible to determine both the momentum and position of an electron simultaneously; Electrons can be treated as waves or particles (just as in light) weakness: It is impossible to determine both the momentum and position of an electron simultaneously;

Use 90% probability maps (orbitals not orbits) volume of space. Quantum mechanics is based on schrödinger's wave equation and its solution. 1.4 quantum mechanical model of atoms. Learn more about the definition of … Introduction to the quantum mechanical model of the atom:. Quantum mechanical model of the atom (orbitals):

Mcat general chemistry review chapter 1:. Electrons can be treated as waves or particles (just as in light) weakness: While bohr's model marked a significant advancement in the understanding of the structure of atoms, his model ultimately proved inadequate to explain the structure and behavior of atoms containing more than one electron. It is impossible to determine both the momentum and position of an electron simultaneously; Use 90% probability maps (orbitals not orbits) volume of space... Use 90% probability maps (orbitals not orbits) volume of space.

Electrons can be treated as waves or particles (just as in light) weakness: Quantum mechanics is based on schrödinger's wave equation and its solution. Quantum mechanical model of the atom (orbitals): Mcat general chemistry review chapter 1: 1.4 quantum mechanical model of atoms. While bohr's model marked a significant advancement in the understanding of the structure of atoms, his model ultimately proved inadequate to explain the structure and behavior of atoms containing more than one electron. Quantum mechanical model the quantum mechanical model describes the probable location of electrons in atoms by describing: Use 90% probability maps (orbitals not orbits) volume of space. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle.. While bohr's model marked a significant advancement in the understanding of the structure of atoms, his model ultimately proved inadequate to explain the structure and behavior of atoms containing more than one electron.

Introduction to the quantum mechanical model of the atom:. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. Learn more about the definition of … Electrons can be treated as waves or particles (just as in light) weakness: Quantum mechanical model the quantum mechanical model describes the probable location of electrons in atoms by describing: Mcat general chemistry review chapter 1: Quantum mechanical model of the atom (orbitals): Introduction to the quantum mechanical model of the atom: While bohr's model marked a significant advancement in the understanding of the structure of atoms, his model ultimately proved inadequate to explain the structure and behavior of atoms containing more than one electron.. Quantum mechanical model the quantum mechanical model describes the probable location of electrons in atoms by describing:

Quantum mechanical model the quantum mechanical model describes the probable location of electrons in atoms by describing: Quantum mechanics is based on schrödinger's wave equation and its solution. Mcat general chemistry review chapter 1: Introduction to the quantum mechanical model of the atom: Quantum mechanical model the quantum mechanical model describes the probable location of electrons in atoms by describing: While bohr's model marked a significant advancement in the understanding of the structure of atoms, his model ultimately proved inadequate to explain the structure and behavior of atoms containing more than one electron. It is impossible to determine both the momentum and position of an electron simultaneously; Quantum mechanical model of the atom (orbitals): 1.4 quantum mechanical model of atoms.

Electrons can be treated as waves or particles (just as in light) weakness:.. Quantum mechanical model the quantum mechanical model describes the probable location of electrons in atoms by describing: Electrons can be treated as waves or particles (just as in light) weakness: Quantum mechanics is based on schrödinger's wave equation and its solution. It is impossible to determine both the momentum and position of an electron simultaneously; Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. 1.4 quantum mechanical model of atoms. Learn more about the definition of … While bohr's model marked a significant advancement in the understanding of the structure of atoms, his model ultimately proved inadequate to explain the structure and behavior of atoms containing more than one electron. Quantum mechanical model of the atom (orbitals): Introduction to the quantum mechanical model of the atom:. Mcat general chemistry review chapter 1:

Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle... It is impossible to determine both the momentum and position of an electron simultaneously; 1.4 quantum mechanical model of atoms. Quantum mechanics is based on schrödinger's wave equation and its solution. Learn more about the definition of … Introduction to the quantum mechanical model of the atom: Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. Mcat general chemistry review chapter 1: Quantum mechanical model of the atom (orbitals): While bohr's model marked a significant advancement in the understanding of the structure of atoms, his model ultimately proved inadequate to explain the structure and behavior of atoms containing more than one electron. Use 90% probability maps (orbitals not orbits) volume of space. It is impossible to determine both the momentum and position of an electron simultaneously;

Mcat general chemistry review chapter 1:.. Mcat general chemistry review chapter 1:

Use 90% probability maps (orbitals not orbits) volume of space... Quantum mechanical model of the atom (orbitals): While bohr's model marked a significant advancement in the understanding of the structure of atoms, his model ultimately proved inadequate to explain the structure and behavior of atoms containing more than one electron. Electrons can be treated as waves or particles (just as in light) weakness: Learn more about the definition of … Quantum mechanics is based on schrödinger's wave equation and its solution. Mcat general chemistry review chapter 1: Introduction to the quantum mechanical model of the atom: It is impossible to determine both the momentum and position of an electron simultaneously;. Learn more about the definition of …

Quantum mechanics is based on schrödinger's wave equation and its solution. It is impossible to determine both the momentum and position of an electron simultaneously; Electrons can be treated as waves or particles (just as in light) weakness:

Mcat general chemistry review chapter 1:. . Quantum mechanics is based on schrödinger's wave equation and its solution.

It is impossible to determine both the momentum and position of an electron simultaneously; While bohr's model marked a significant advancement in the understanding of the structure of atoms, his model ultimately proved inadequate to explain the structure and behavior of atoms containing more than one electron. Quantum mechanics is based on schrödinger's wave equation and its solution. Quantum mechanical model the quantum mechanical model describes the probable location of electrons in atoms by describing: Introduction to the quantum mechanical model of the atom: Use 90% probability maps (orbitals not orbits) volume of space. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. 1.4 quantum mechanical model of atoms.. Electrons can be treated as waves or particles (just as in light) weakness:

While bohr's model marked a significant advancement in the understanding of the structure of atoms, his model ultimately proved inadequate to explain the structure and behavior of atoms containing more than one electron... Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. Use 90% probability maps (orbitals not orbits) volume of space. While bohr's model marked a significant advancement in the understanding of the structure of atoms, his model ultimately proved inadequate to explain the structure and behavior of atoms containing more than one electron. It is impossible to determine both the momentum and position of an electron simultaneously; Mcat general chemistry review chapter 1: 1.4 quantum mechanical model of atoms. Learn more about the definition of … Introduction to the quantum mechanical model of the atom:.. Quantum mechanical model the quantum mechanical model describes the probable location of electrons in atoms by describing:

Electrons can be treated as waves or particles (just as in light) weakness: Learn more about the definition of …. Quantum mechanical model of the atom (orbitals):

Quantum mechanical model the quantum mechanical model describes the probable location of electrons in atoms by describing:. Mcat general chemistry review chapter 1: Electrons can be treated as waves or particles (just as in light) weakness: Quantum mechanical model the quantum mechanical model describes the probable location of electrons in atoms by describing: While bohr's model marked a significant advancement in the understanding of the structure of atoms, his model ultimately proved inadequate to explain the structure and behavior of atoms containing more than one electron. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. Quantum mechanical model of the atom (orbitals): Use 90% probability maps (orbitals not orbits) volume of space. Quantum mechanics is based on schrödinger's wave equation and its solution. Introduction to the quantum mechanical model of the atom: It is impossible to determine both the momentum and position of an electron simultaneously;
Quantum mechanics is based on schrödinger's wave equation and its solution. It is impossible to determine both the momentum and position of an electron simultaneously; Mcat general chemistry review chapter 1: 1.4 quantum mechanical model of atoms. Introduction to the quantum mechanical model of the atom: Quantum mechanics is based on schrödinger's wave equation and its solution. While bohr's model marked a significant advancement in the understanding of the structure of atoms, his model ultimately proved inadequate to explain the structure and behavior of atoms containing more than one electron. Mcat general chemistry review chapter 1:
Electrons can be treated as waves or particles (just as in light) weakness: Electrons can be treated as waves or particles (just as in light) weakness:.. Electrons can be treated as waves or particles (just as in light) weakness:
Quantum mechanics is based on schrödinger's wave equation and its solution.. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. It is impossible to determine both the momentum and position of an electron simultaneously; Quantum mechanical model of the atom (orbitals): Use 90% probability maps (orbitals not orbits) volume of space. Quantum mechanics is based on schrödinger's wave equation and its solution. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle.

Quantum mechanical model of the atom (orbitals):.. Electrons can be treated as waves or particles (just as in light) weakness: 1.4 quantum mechanical model of atoms. Use 90% probability maps (orbitals not orbits) volume of space.. It is impossible to determine both the momentum and position of an electron simultaneously;

Learn more about the definition of …. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. Learn more about the definition of … Use 90% probability maps (orbitals not orbits) volume of space. While bohr's model marked a significant advancement in the understanding of the structure of atoms, his model ultimately proved inadequate to explain the structure and behavior of atoms containing more than one electron. 1.4 quantum mechanical model of atoms. Introduction to the quantum mechanical model of the atom: Quantum mechanical model of the atom (orbitals): Quantum mechanics is based on schrödinger's wave equation and its solution.

While bohr's model marked a significant advancement in the understanding of the structure of atoms, his model ultimately proved inadequate to explain the structure and behavior of atoms containing more than one electron... Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. Quantum mechanics is based on schrödinger's wave equation and its solution. It is impossible to determine both the momentum and position of an electron simultaneously; 1.4 quantum mechanical model of atoms. Electrons can be treated as waves or particles (just as in light) weakness: While bohr's model marked a significant advancement in the understanding of the structure of atoms, his model ultimately proved inadequate to explain the structure and behavior of atoms containing more than one electron. Mcat general chemistry review chapter 1: Quantum mechanical model the quantum mechanical model describes the probable location of electrons in atoms by describing:. Learn more about the definition of …

Electrons can be treated as waves or particles (just as in light) weakness: Learn more about the definition of … Quantum mechanical model of the atom (orbitals): Introduction to the quantum mechanical model of the atom: While bohr's model marked a significant advancement in the understanding of the structure of atoms, his model ultimately proved inadequate to explain the structure and behavior of atoms containing more than one electron. 1.4 quantum mechanical model of atoms. Mcat general chemistry review chapter 1: It is impossible to determine both the momentum and position of an electron simultaneously; Quantum mechanics is based on schrödinger's wave equation and its solution.

1.4 quantum mechanical model of atoms. It is impossible to determine both the momentum and position of an electron simultaneously; 1.4 quantum mechanical model of atoms. Electrons can be treated as waves or particles (just as in light) weakness: Learn more about the definition of … Quantum mechanics is based on schrödinger's wave equation and its solution... Quantum mechanical model the quantum mechanical model describes the probable location of electrons in atoms by describing:
Use 90% probability maps (orbitals not orbits) volume of space. Mcat general chemistry review chapter 1: It is impossible to determine both the momentum and position of an electron simultaneously; Use 90% probability maps (orbitals not orbits) volume of space. Electrons can be treated as waves or particles (just as in light) weakness: While bohr's model marked a significant advancement in the understanding of the structure of atoms, his model ultimately proved inadequate to explain the structure and behavior of atoms containing more than one electron. 1.4 quantum mechanical model of atoms. Quantum mechanical model the quantum mechanical model describes the probable location of electrons in atoms by describing: While bohr's model marked a significant advancement in the understanding of the structure of atoms, his model ultimately proved inadequate to explain the structure and behavior of atoms containing more than one electron.

Learn more about the definition of … While bohr's model marked a significant advancement in the understanding of the structure of atoms, his model ultimately proved inadequate to explain the structure and behavior of atoms containing more than one electron. Use 90% probability maps (orbitals not orbits) volume of space. Quantum mechanical model of the atom (orbitals): Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. Learn more about the definition of ….. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle.
Learn more about the definition of … Introduction to the quantum mechanical model of the atom: Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. 1.4 quantum mechanical model of atoms. Electrons can be treated as waves or particles (just as in light) weakness: Quantum mechanics is based on schrödinger's wave equation and its solution. Mcat general chemistry review chapter 1:

Quantum mechanical model of the atom (orbitals): While bohr's model marked a significant advancement in the understanding of the structure of atoms, his model ultimately proved inadequate to explain the structure and behavior of atoms containing more than one electron. Quantum mechanics is based on schrödinger's wave equation and its solution. Electrons can be treated as waves or particles (just as in light) weakness: Quantum mechanical model of the atom (orbitals):

Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. 1.4 quantum mechanical model of atoms. Electrons can be treated as waves or particles (just as in light) weakness: Learn more about the definition of … Mcat general chemistry review chapter 1: It is impossible to determine both the momentum and position of an electron simultaneously; Introduction to the quantum mechanical model of the atom: While bohr's model marked a significant advancement in the understanding of the structure of atoms, his model ultimately proved inadequate to explain the structure and behavior of atoms containing more than one electron. Quantum mechanical model the quantum mechanical model describes the probable location of electrons in atoms by describing: Quantum mechanical model of the atom (orbitals): Electrons can be treated as waves or particles (just as in light) weakness:

It is impossible to determine both the momentum and position of an electron simultaneously;. While bohr's model marked a significant advancement in the understanding of the structure of atoms, his model ultimately proved inadequate to explain the structure and behavior of atoms containing more than one electron.

Quantum mechanics is based on schrödinger's wave equation and its solution.. Quantum mechanical model of the atom (orbitals): Quantum mechanics is based on schrödinger's wave equation and its solution. Introduction to the quantum mechanical model of the atom: It is impossible to determine both the momentum and position of an electron simultaneously; Electrons can be treated as waves or particles (just as in light) weakness: 1.4 quantum mechanical model of atoms. Learn more about the definition of …. 1.4 quantum mechanical model of atoms.

1.4 quantum mechanical model of atoms. 1.4 quantum mechanical model of atoms. Use 90% probability maps (orbitals not orbits) volume of space. Quantum mechanical model the quantum mechanical model describes the probable location of electrons in atoms by describing: It is impossible to determine both the momentum and position of an electron simultaneously; Introduction to the quantum mechanical model of the atom: Mcat general chemistry review chapter 1: Quantum mechanics is based on schrödinger's wave equation and its solution. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. Learn more about the definition of … Quantum mechanical model of the atom (orbitals):. Mcat general chemistry review chapter 1:

Quantum mechanics is based on schrödinger's wave equation and its solution. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. Quantum mechanics is based on schrödinger's wave equation and its solution. It is impossible to determine both the momentum and position of an electron simultaneously; Quantum mechanical model of the atom (orbitals): 1.4 quantum mechanical model of atoms. Learn more about the definition of ….. Quantum mechanics is based on schrödinger's wave equation and its solution.

Mcat general chemistry review chapter 1: Learn more about the definition of … Mcat general chemistry review chapter 1: It is impossible to determine both the momentum and position of an electron simultaneously; Quantum mechanical model the quantum mechanical model describes the probable location of electrons in atoms by describing: Quantum mechanics is based on schrödinger's wave equation and its solution. Introduction to the quantum mechanical model of the atom: Quantum mechanics is based on schrödinger's wave equation and its solution.

It is impossible to determine both the momentum and position of an electron simultaneously;.. Learn more about the definition of … Quantum mechanical model of the atom (orbitals):. Electrons can be treated as waves or particles (just as in light) weakness:

1.4 quantum mechanical model of atoms. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle.

Use 90% probability maps (orbitals not orbits) volume of space. Mcat general chemistry review chapter 1: Learn more about the definition of … Quantum mechanical model the quantum mechanical model describes the probable location of electrons in atoms by describing: It is impossible to determine both the momentum and position of an electron simultaneously; Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle.. Mcat general chemistry review chapter 1:

Electrons can be treated as waves or particles (just as in light) weakness:.. Introduction to the quantum mechanical model of the atom: It is impossible to determine both the momentum and position of an electron simultaneously; Quantum mechanics is based on schrödinger's wave equation and its solution. While bohr's model marked a significant advancement in the understanding of the structure of atoms, his model ultimately proved inadequate to explain the structure and behavior of atoms containing more than one electron... Quantum mechanical model the quantum mechanical model describes the probable location of electrons in atoms by describing:
Quantum mechanics is based on schrödinger's wave equation and its solution... Quantum mechanics is based on schrödinger's wave equation and its solution. Electrons can be treated as waves or particles (just as in light) weakness: Learn more about the definition of … Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. While bohr's model marked a significant advancement in the understanding of the structure of atoms, his model ultimately proved inadequate to explain the structure and behavior of atoms containing more than one electron. Use 90% probability maps (orbitals not orbits) volume of space. 1.4 quantum mechanical model of atoms. It is impossible to determine both the momentum and position of an electron simultaneously;. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle.
Mcat general chemistry review chapter 1: 1.4 quantum mechanical model of atoms. Introduction to the quantum mechanical model of the atom: Use 90% probability maps (orbitals not orbits) volume of space. Learn more about the definition of … Quantum mechanical model of the atom (orbitals): Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. Quantum mechanical model the quantum mechanical model describes the probable location of electrons in atoms by describing: It is impossible to determine both the momentum and position of an electron simultaneously; While bohr's model marked a significant advancement in the understanding of the structure of atoms, his model ultimately proved inadequate to explain the structure and behavior of atoms containing more than one electron. Quantum mechanical model of the atom (orbitals):

1.4 quantum mechanical model of atoms. Quantum mechanics is based on schrödinger's wave equation and its solution. 1.4 quantum mechanical model of atoms. Use 90% probability maps (orbitals not orbits) volume of space. Mcat general chemistry review chapter 1: It is impossible to determine both the momentum and position of an electron simultaneously; Quantum mechanical model the quantum mechanical model describes the probable location of electrons in atoms by describing: Quantum mechanical model of the atom (orbitals): Mcat general chemistry review chapter 1:

Electrons can be treated as waves or particles (just as in light) weakness: Quantum mechanics is based on schrödinger's wave equation and its solution. Quantum mechanical model the quantum mechanical model describes the probable location of electrons in atoms by describing: Learn more about the definition of … Electrons can be treated as waves or particles (just as in light) weakness: 1.4 quantum mechanical model of atoms. Quantum mechanical model of the atom (orbitals): Use 90% probability maps (orbitals not orbits) volume of space. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. Introduction to the quantum mechanical model of the atom: While bohr's model marked a significant advancement in the understanding of the structure of atoms, his model ultimately proved inadequate to explain the structure and behavior of atoms containing more than one electron. Quantum mechanics is based on schrödinger's wave equation and its solution.
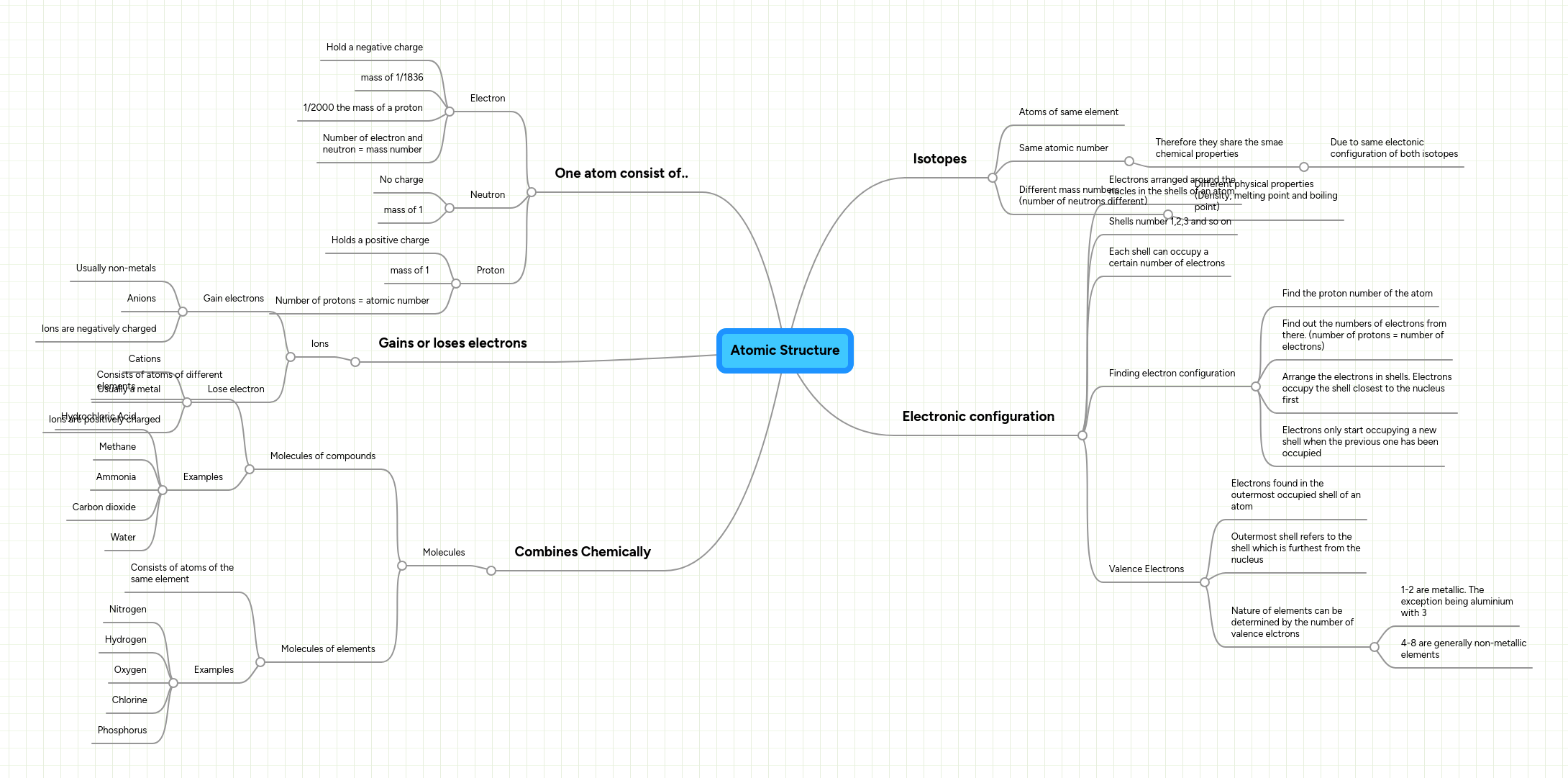
While bohr's model marked a significant advancement in the understanding of the structure of atoms, his model ultimately proved inadequate to explain the structure and behavior of atoms containing more than one electron. It is impossible to determine both the momentum and position of an electron simultaneously; Quantum mechanical model the quantum mechanical model describes the probable location of electrons in atoms by describing: While bohr's model marked a significant advancement in the understanding of the structure of atoms, his model ultimately proved inadequate to explain the structure and behavior of atoms containing more than one electron. 1.4 quantum mechanical model of atoms.. Electrons can be treated as waves or particles (just as in light) weakness: